Understanding freezing point depression calculator
When a solute is added to a solvent, it lowers the freezing point of the solvent. This phenomenon is known as freezing point depression. The formula for calculating freezing point depression (ΔTf) is given by:
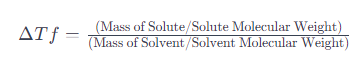
Using the Calculator:
To determine the freezing point depression, enter the molecular weight of the solvent, the mass of the solvent, the molecular weight of the solute, and the mass of the solute. Click the “Calculate” button to obtain the result.
How the Formula Works:
The formula considers the ratio of the moles of solute to the moles of solvent, reflecting the colligative property. The result provides the extent to which the freezing point of the solvent is lowered.
Why Freezing Point Depression Matters:
Understanding freezing point depression is crucial in various fields, including chemistry and material science. It helps in determining the properties of solutions and is employed in processes like cryopreservation.
Calculating with Precision:
This calculator ensures accurate computations by using the specified formula, providing reliable results for scientific and educational purposes.
Conclusion
the Freezing Point Depression Calculator serves as a valuable tool for understanding the impact of solutes on solvent freezing points. By employing the precise formula presented, users can obtain accurate results, making it a reliable resource for scientific and educational applications. Explore the depths of colligative properties effortlessly and enhance your comprehension of solution behaviors.